
Interest in supercritical fluid processing of polymers continues to grow, and many purification, fractionation, and polymerization applications have emerged. A significant motivation for applying this technology to polymers is the increasing performance demands required of polymer products coupled with the technical limitations of more traditional purification and fractionation methods. With increasing scrutiny of industrial solvents, supercritical fluid technology—especially using carbon dioxide—is receiving widespread attention as an environmentally conscious method for replacing various organic solvents used in industrial operations.
Supercritical fluid (SCF) technology has proven success in the following industrial applications:

Feasibility Studies —These are short, fast-response feasibility studies to demonstrate that processing with supercritical fluids can achieve your desired goals, both technically and economically.
Contract R&D—R&D projects are designed to achieve your goals—from creating a new product, improving an existing one, recovering a by-product, or other purposes.
Toll Processing—Our ability to process 1,000 to 100,000 kg quantities enables you to evaluate supercritical fluids for their specific needs without investing early stage and high-risk capital
Technology Licensing—Phasex can develop technology for supercritical separations to be carried out as a part of a company’s own manufacturing train.
Reactive monomers, especially those with very low vapor pressure, are inherently difficult to process by high vacuum or molecular distillation. Supercritical fluids purify reactive monomers by extracting odors and color bodies without polymerization problems. Phasex has also applied SCF technology to fractionate specialty polymers for improved performance. Additionally, Phasex has exploited the unique dissolving powers of SCFs to reclaim virgin-like polymer fractions from plastic waste streams.
.jpg)
Residual raw materials and objectional by-products are readily extracted from polymers. Additionally, polymers can be fractionated by molecular weight (MW), chemical composition, or crystallinity to enhance product performance, to characterize structure-property behavior, or for use as calibration standards.
Phasex has been instrumental in developing many of the supercritical fluid processes currently in production or advanced development:
A few examples are presented below to demonstrate the breadth of polymer applications using supercritical fluids
It is possible to tailor the performance of polymers by modifying certain properties, such as molecular weight, polydispersity, or crystallinity via supercritical fluid extraction or fractionation. For example, the undesirably high viscosity of a polymer can be reduced by separating the very high molecular weight species from the polymer via supercritical fluid fractionation.
Similarly, undesired low molecular weight or cyclic species in a silicone polymer, which can migrate in some high temperature applications, can be removed by SCF extraction. Because their dissolving power can be fine-tuned, often to high degree of selectivity, supercritical fluids can separate polymers by molecular weight, which, as suggested earlier, can enhance their performance
An example is presented to demonstrate the effectiveness of SCF fractionation in preparing narrow polydispersity polymer fractions which are useful in characterizing structure-property relations, elucidating reaction kinetics, and even as calibration standards.
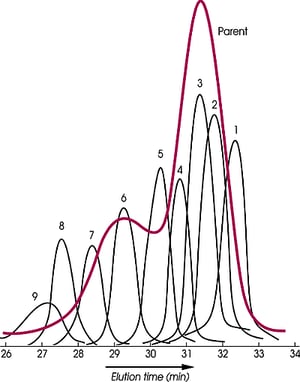
For the exact determination of molecular weight by SEC, narrow standards of the polymer being analyzed must be used, but they are not generally available, especially for polymers like the perfluoropolyethers (Krytox®, Fomblin®), high molecular weight silicones, and polyethylene and its copolymers
The polydispersity of the parent polymer shown is 1.87. It has been reduced to an average of 1.08 for the nine fractions. Molecular distillation cannot carry out the fractionation of this polymer because its vapor pressure is too low, but supercritical fluids have been effective in producing narrow standards of the high molecular weight (>8,800) perfluoropolyether.
The properties of supercritical fluids can also be manipulated so as to fractionate polymers by, for example, crystallinity. Fractionation of polyethylene by molecular weight and side chain branching is another example presented here of the advantages offered by supercritical fluid processing
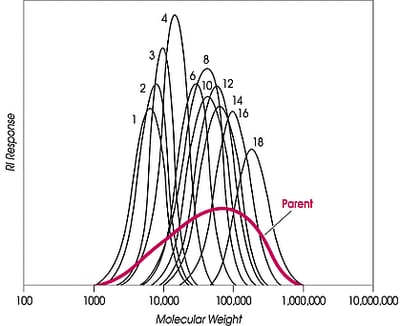
Narrow fractions of polyethylene and its copolymers are desired for many reasons, e.g., for GPC calibration standards, properties evaluation, kinetics studies, or catalyst performance analysis. Generally, the fractions are not commercially available. For the specific case of GPC standards, hydrogenated polybutadiene is sometimes used, but it is not a good model for commercial polyethylene, such as LDPE, HDPE, LLDPE, and certainly not for experimental copolymers; there is, for example, no short, and long-chain branching on hydrogenated polybutadiene, and although molecular weight ranges of HDPE or LLDPE can be matched, the hydrodynamic volume cannot be.
Quantities of very small (mg) size can be obtained by GPC fractionation or by anti-solvent methods, and a laboratory process called TREF (temperature rising elution fractionation) can produce small quantities of polyethylene separated by crystallinity; however, producing preparative amounts requires many liters of solvent to process even a 5g charge.At a preparative bench scale, fractionation with supercritical fluids produces large quantities of narrow MW fractions. Additionally, CITREF (critical isobaric temperature rising elution fractionation), Phasex Corporation’s supercritical variant of TREF, can separate ethylene polymers and copolymers by side chain branching and chemical composition, again producing large fractions. The HDPE described here was fractionated by the process Phasex terms increasing pressure profiling.
Click here to see the MN, MW, and Polydispersity Fraction Table.
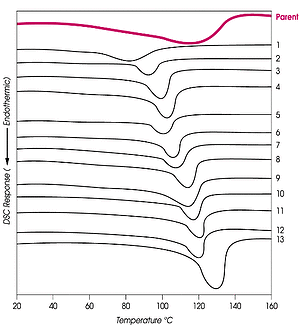
Supercritical fluids can also fractionate polyethylene and its copolymers (e.g., acrylate, methacrylate, acrylic acid, vinyl acetate) by crystallinity/side chain branching/chemical composition. The process Phasex terms CITREF can, like pressure profiling, produce large fractions for fundamental studies, polymer properties determination, or catalyst performance evaluation. A commercial LLDPE was fractionated by amount of side branching. CITREF has separated the LLDPE, not by narrow MW, but by crystallinity (melting point).
Click here to see MN and MW for all fractions.
DSC thermograms of the 13 LLDPE fractions separated by side chain branching are shown in the figure on the right and are compared with the DSC of the parent polymer. The narrow transition range and the increasing transition temperature of each fraction obtained by CITREF are readily seen. The CITREF fractions of polyethylene are useful for the overall evaluation of the polymerization process, the narrow fractions providing quantitative information on kinetic profiles, and catalyst life and performance (mols monomer/mol of active catalyst).
Other polyolefins and copolymers produced by virtually any catalyst technology can be readily processed with supercritical fluids by pressure profiling, for molecular weight distribution, and by CITREF, for crystal unity and chemical composition distribution
Polyolefins and copolymers fractionated by molecular weight, side chain branching, and chemical composition—at the kilogram scale—can facilitate your determination of polymer structure/property relationships and polymerization catalyst performance. Let Phasex fractionate your polymers for kinetic studies, catalyst life/performance evaluation, properties determination, and new product development(NPD)
Many medical devices that are in contact with the body or body fluids or that are surgically implanted in the body are composed of silicone polymers because of their biocompatibility. The silicone parts are lightly cross linked to retain structure, but the cyclic byproducts present in the silicone polymer are not incorporated into the matrix; thus, they can migrate. Since the volatility of the cyclics is so low, high temperature vacuum or nitrogen stripping is ineffective. Organic liquid extraction can be effective in removing the interfering species, but the issue of residual solvents in the devices then becomes a concern. Supercritical fluid extraction of residual cyclics from medical devices is attractive especially because the purification process cannot be reasonably carried out by any other technique. Cyclics and low molecular weight oligomers content can be as high as 4 wt%, and extraction with supercritical CO2 can reduce the level of these species to less than 10ppm. Several examples of medical products that have been extracted using SCFs include aorta and other arterial grafts, neuro-shunt lines, and catheters.
Supercritical fluids have also been used to extract other medical; and ocular materials, for example, to purify methacrylate functionality silicone macromonomers that are used in the manufacture of soft contact lenses. Because these macromonomers are very heat labile and have very low volatility (and thus require high temperature to purify them even under high vacuum), they are virtually impossible to purify by any traditional process.
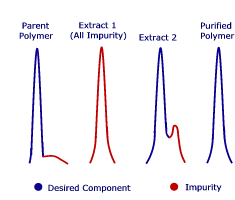
Reactive monomers are inherently difficult to process by traditional methods. SCFs offer a useful alternative for extracting; for example, odors from an acrylate monomer. The HPLC traces shown demonstrate that even minute quantities of impurities can be readily extracted from a temperature-sensitive methacrylic monomer.
Extraction and fractionation are the most common operations using supercritical fluids, but the process can be reversed to deposit materials, for example, into a porous or polymeric substrate. In this application, a supercritical fluid is used to convey an organic compound into micropores of a substrate, the pressure/temperature then reduced to bring about precipitation in the micropores. With polymeric substrates, the SCF first swells the polymer then conveys and deposits a compound in the matrix. Monomers and polymers can also be impregnated into a porous substrate, but the procedure is a bit more complex, viz., the pressure/temperature must be selected so as to dissolve the entire polymer (not just the low molecular species). Because the dissolving power of SCFs can be adjusted over wide ranges, the conditions can be manipulated so as to dissolve and deposit the entire polymer homogeneously.
With the rapid evolution of products in the microelectronics industry, there is an increasing need for higher purity materials and safer, more efficient solvents, and supercritical fluids are proving effective in satisfying these needs in diverse photoresist applications ranging from polymer purification and fractionation to image developing. SCFs have been applied to improving the performance of photoresist polymers by purification and/or fractionation. Specialty polysiloxane and polysilane polymers that are being developed as photoresists have broad molecular weight distribution resulting in a variable sensitivity to radiation at 248-254 nm wavelength. Sensitivity can be controlled by using near-monodisperse fractions of these polymers in resist applications, but there is no traditional synthesis method that can produce monodisperse polysilanes or polysiloxanes; SCF fractionation overcomes this problem.
Supercritical fluids have also been applied as developers for photoresist imaging; they eliminate problems associated with organic liquid developers, such as swelling and image distortion and they minimize solvent waste. Furthermore, utilization of SCFs for photoresist imaging is applicable to a number of new polymer systems under development as next-generation resists. SCF imaging has been demonstrated with several polymer systems including silanes and siloxanes, fluorinated methacrylates, and siloxane-modified methacrylates for creating positive or negative tone images
We list the government-funded programs awarded to Phasex Corporation. They illustrate our breadth of experience and diverse fields of research. More than $3 Million of SBIR grants has resulted in the generation of the broad knowledge base that provides a competitive advantage for Phasex and for its customers. This knowledge base reduces development time on any new application, minimizes development expenses, and decreases time to market.
The contracts are listed chronologically, starting with the first SBIR contract, an NSF-funded polymers fractionation program, in 1983. Research on this program led to the first two technical publications of Phasex in 1984, joint publications with researchers from Virginia Polytechnical Institute and State University. (The papers are listed in the publications section). Many of the government-funded programs resulted in publications.
National Science Foundation
(CPE-8361087)
Field: Polymer Processing
Program Title: Research on a New Process to Fractionate Polymers
National Institutes of Health
(N43-HB-57005)
Field: Polymer Processing
Program Title: Supercritical Fluid Fractionation of Pluronic F68 (for Substitute Blood Formulation)
Air Force Office of Scientific Research/DARPA
(F4920-85-C-0053)
Field: Advanced Materials
Program Title: Infiltration, A New Process for Densification of Ceramics
National Science Foundation
(ISI-8509931)
Field: Polymer Processing
Program Title: Research on a New Process to Fractionate Polymers (Phase II)
Department of Energy
(DE 86012336)
Field: Wastewater Extraction
Program Title: Supercritical Extraction of Phenolic Wastewater
Ballistic Research Laboratory
(DAAA15-86-C-0079)
Field: Recrystallization
Program Title: Exploratory Development on a New Process to Produce Improved RDX Crystals
National Science Foundation
(ISI-8660823)
Field: Recrystallization
Program Title: Supercritical Fluid Nucleation: An Improved Ultra-Fine Particle Formation Process
Air Force Armament Laboratory
(F08635-87-C-0346)
Field: Recrystallization
Program Title: Exploratory Development on a New Nitroguanidine Recrystallization Process
US Army Research Office/BRL Scientific Services Program
(DAAL03-86-D-0001)
Field: Recrystallization/Purification
Program Title: Gas Anti-Solvent Recrystallization: Application to the Separation and Subsequent Processing of RDX and HMX
National Science Foundation
(ISI-8760545)
Field: Separation
Program Title: Single Phase Enrichment of Supercritical Fluid Mixtures
Air Force Armament Laboratory
(F08635-89-C-0066)
Field: Recrystallizatio
Program Title: Exploratory Development on a New Nitroguanidine Recrystallization Process using Habit Modifiers
Astronautics Laboratory
(F04611-89-C-00420)
Field: Polymer Processing
Program Title: Supercritical Fluid Processing of Propellant Polymers
National Science Foundation
(CTS 8900122-subcontract)
Field: Polymer Processing
Program Title: Supercritical Fluid-Crystal Phase Fractionation of Polymers: Polyethylene-Propane
Air Force Armament Laboratory
(F08635-89-C-0193)
Field: Recrystallization
Program Title: Exploratory Development on a New Nitroguanidine Recrystallization Process (Phase II)
Defense Advanced Research Projects Agency (through Army Missile Command)
(DAAH1-90-C-0732)
Field: Extraction/Reclamation
Program Title: Supercritical Fluid Processing of a Single-Base Propellant and a Nitramine-Base Munition
Wright Research and Development Center
(F33615-90-C-2075)
Field: Purification/Fractionation
Program Title: Development of JP-8 with Improved Thermal Oxidiative Stability
Arnold Engineering and Development Center
(F40600-90-C-0021)
Field: Recrystallization
Program Title: Development of a Submicron Monodisperse Aerosol Generator for Laser Doppler Velocimetry
Ballistic Missile Organization
(F04704-91-C-0033)
Field: Solvent Substitution
Program Title: Freon (CFC-113) solvent Replacement: A New Process for Precision Parts Cleaning
Chemical and Biological Defense Agency Procurement Division
(DAAA15-92-C-0037)
Field: Recrystallization/Dissemination
Program Title: Supercritical Fluid Nucleation for Improved Dissemination of Powdered Materials
Naval Surface Warfare Center
(N00174-92-C-0050)
Field: Extraction/Reclamation/Recrystallization
Program Title: Processing of Energetic Materials with Supercritical Fluids
Strategic Defense Initiative Organization
(N60921-92-C-0140)
Field: Advanced Materials: Infiltration
Program Title: Improved Method for Producing Silicon Carbide/Silicon Carbide Composites
Department of Energy/Westinghouse
(MBH-SVV-273110)
Field: Extraction/Remediation
Program Title: Supercritical Extraction (SFE) Remediation of Hanford Contaminated Soils
Ballistic Missile Organization
(F04704-92-C-0036)Field:
Solvent Substitution
Program Title: Precision Parts Cleaning: Freon Replacement Technology (Phase II)
Air Force Office of Scientific Research
(F49620-92-C-0036)
Field: Advanced Materials: Infiltration
Program Title: Improved Oxidation Resistance of 3D Carbon/Carbon Composites
U.S. Army CBDCOM
(DAAM01-94-C-0052)
Field: Extraction/Decontamination
Program Title: Decontamination of Delicate Military Equipment Using Supercritical Fluid Technology
U.S. Air Force
(F04611-94-C-0081)
Field: Recrystallization
Program Title: Phase Stabilization of Ammonium Nitrate for Environmentally Conscious Propellants: Gas Anti-Solvent Recrystallization
Office of Naval Research (Technology Reinvestment Program)
(N00014-94-C-0235)
Field: Solvent Substitution
Program Title: Environmentally Acceptable Photoresist Processing for Integrated Circuit Manufacturing
michele.mersicano@robertet.com
125 Flagship Drive
North Andover, MA 01845