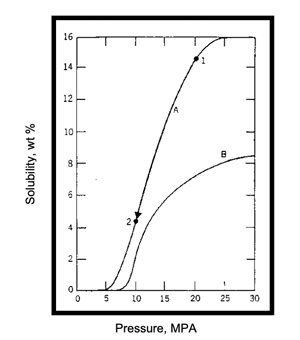
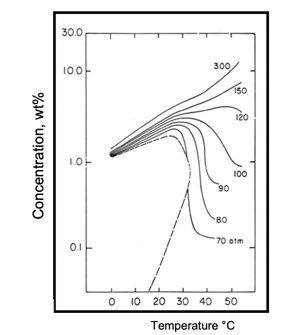
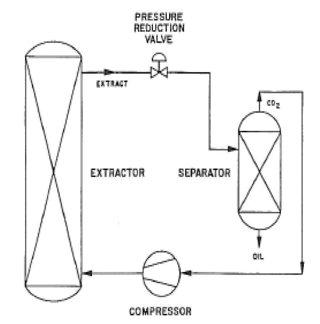
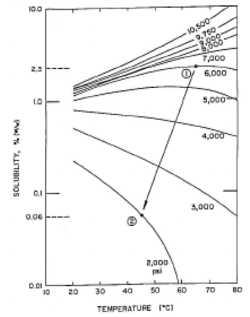
The process schematic (a) shows a simplified industrial supercritical CO2 extraction process. With reference to the solubility map (b), supercritical CO2 at selected pressure-temperature conditions of 300atm, 55°C (Point 1) is passed through the extraction vessel that has been charged with naphthalene (in admixture with some other solid). As CO2 passes (percolates) through the vessel contents, naphthalene is dissolved to its solubility limit of 15 wt% and is extracted from the mixture. The supercritical solution (of CO2 and naphthalene) is passed through the pressure reduction valve and dropped in pressure to 90atm (due to thermodynamic phenomena the CO2 temperature drops to 35°C – Point 2). At 90atm, 35°C the solubility of naphthalene is only ~ 2%; therefore, more than 90% of the naphthalene “drops out like a snow” in the separator as it is no longer soluble in CO2 at these new conditions. The precipitated naphthalene remains in the separator as the CO2 gas steam exits to the compressor for re-use (recompressed) for further extraction. This cycle continuing until all the naphthalene is extracted from the vessel and precipitated in the separator.
This example described a process to extract naphthalene from a mixture. This same process can be applied to extracting an active compound from a botanical or plant-based matrix. The resulting extracted product will have no residual solvent as CO2, is not considered a volatile organic compound (VOC), and is a true gas in ambient pressure and temperature.
Overview of Advantages
Careful consideration of the supercritical CO2 extraction process results in understanding many advantages over traditional solvent extraction process. These advantages are listed and described below:
- No Solvent Residue: As explained above in our supercritical CO2 extraction process description, there remains no solvent residue in the final extracted product.
- Organic Certifiable Process: Supercritical CO2 extraction can be considered an organic process. Using synthetic, petroleum-based, and non-natural traditional solvents in an extraction process will disqualify the resulting final product extract from being able to be certified USDA organic. An extraction process using only CO2 will not prohibit the final product from being certified USDA Organic.
- Superior Product: The mild extraction temperatures used to collect the product in a supercritical CO2 extraction process as compared to the high temperatures used in distillation and solvent vaporization processes may lead to superior and more robust flavor profiles and full-spectrum final product extracts.
- Environmentally Conscious Process: The CO2 used in a supercritical CO2 extraction process is captured from natural fermentation processes. No CO2 is generated during the extraction process and much of the CO2 gas is used in recycle mode to reduce CO2 emissions to the atmosphere. There is no net gain of CO2 from the extraction process—it is a carbon neutral process. CO2 extraction processes can replace petroleum-based solvent extraction processes, and thus, significantly reduce the carbon footprint required to generate those petroleum-based solvents.
- Flammability/Hazardous – Safety Concerns: CO2 is a non-flammable and non-hazardous gas. Moving to a supercritical CO2 extraction process eliminates any flammability concerns and employs the use of a non-hazardous extracting medium. All petroleum-based solvents are flammable, and most are considered hazardous materials. For this reason, the spent biomass from a supercritical CO2 extraction process can typically be considered non-hazardous material ready for solid landfill waste or some other green agricultural end use, whereas, those spent biomasses from a petroleum-based solvent extraction process must go through rigorous de-solventizaton processes otherwise be categorized as hazardous materials
Supercritical CO2 Extraction Applications: Natural Products
A common application of the advanced supercritical CO2 extraction method is with the extraction of natural products. Specific to natural products extractions, here is an overview of the process and benefits customers can expect.
 Process
Process
- Supercritical CO2 extraction involves the use of carbon dioxide in its supercritical state as a solvent. In this state, CO2 exhibits both liquid and gas-like properties, making it an excellent choice for extraction.
- The process begins by placing the natural product, such as herbs, plants, or botanicals, into an extraction chamber.
- Carbon dioxide is then pumped into the chamber under high pressure and temperature, causing it to reach a supercritical state.
- The supercritical CO2 interacts with the natural product, dissolving the desired compounds.
- The CO2 and extracted compounds are then passed into a separator, where pressure is lowered, allowing the CO2 to evaporate and separate from the extracted product.
- The final product obtained after the CO2 evaporation is a pure and concentrated extract, free from any residual solvents.
Benefits
Enhanced Extraction Efficiency
- Supercritical CO2 extraction offers higher extraction efficiency compared to conventional methods. It can selectively target and extract specific compounds, leaving behind unwanted impurities.
- The control over pressure and temperature in this method allows for the precise extraction of desired components, minimizing the loss of volatile compounds.
Purity and Safety
- Supercritical CO2 extraction is a completely solvent-free process. Unlike traditional solvent-based methods, there is no risk of residue from solvents contaminating the final extract.
- The end product is pure and free from any harsh chemicals, ensuring the safety and quality of the extract.
Environmentally Friendly
- Carbon dioxide used in the process is a natural and non-toxic substance. It is abundant in the atmosphere and can be easily recycled, making it an environmentally friendly choice.
- Unlike other extraction methods, supercritical CO2 extraction does not contribute to air pollution or water contamination.
Versatility
- Supercritical CO2 extraction is versatile and can be applied to a wide range of natural products. It is commonly used for extracting essential oils, flavors, fragrances, herbal extracts, and cannabinoids from cannabis.
- The process allows for customization based on the desired outcome, such as adjusting pressure and temperature to target specific compounds.
Supercritical CO2 extraction offers a superior and sustainable method of extracting natural products. Its efficient extraction, purity, and environmental friendliness make it highly beneficial for both prospective and existing customers. This method can meet your extraction needs and deliver high-quality extracts for various applications.
Phasex processes a variety of natural product extracts that are used in diverse applications, some of which include:
- Nutraceutical ingredients
- Dietary supplements
- Topical or cosmetic ingredients
- Health supplements
- Food and beverage ingredients
- Pet food ingredients
- Flavor concentration
Supercritical fluids are widely preferred for extraction, purification, recrystallization, and fractionation operations in many industries. Phasex has run thousands of toll processing projects in over four decades—many of those for natural products. This technology is used to process hundreds of millions of pounds of coffee, tea, and hops annually. Supercritical CO2 extraction is also gaining in herbal and botanical extracts, vitamins, and supplements industries—the fastest growing segments of the food industry today—as it is synonymous with the highest purity and quality.
Increasing scrutiny of organic solvents and the demand for improved nutraceutical and natural products have led to the evaluation of alternative and improved extraction methods. Supercritical CO2 concentrate active compounds: astaxanthin from a from microalgae, paclitaxel from yew needles, lycopene from tomato skins, and terpenes form cilantro. We can fractionate EPA/DHA from fish oils and algae, helping nutraceutical companies to provide healthy fat ratios, as well, essential oils from cedarwood and wormwood. Supercritical CO2 extraction is the most efficient separation method for the dietary supplements market; it is capable of providing the highest purities and concentrations attainable. Higher extraction efficiencies increase product concentration and yield, and most important, these superior quality extracts are free of solvent residues.
Phasex’s proprietary extraction process helps maximize your product yields, minimize your contamination, and ensure the highest purity and quality with supercritical fluids.
Supercritical Fluid Chromatography: Process and Importance of Selectivity
Supercritical fluid chromatography (SFC) is a separation technique that uses supercritical fluids as the mobile phase in the chromatographic process. We’ll discuss the process of SFC and why selectivity of particular substances is important and possible.
Process
- SFC involves a stationary phase made up of a packed column, usually filled with a highly polar material.
- The mobile phase is a supercritical fluid (Phasex uses CO2 ), which is pumped into the column.
- The sample is introduced into the mobile phase, and the supercritical fluid carrying the sample is passed through the stationary phase of the column.
- As the compounds in the sample interact with the highly polar stationary phase, the various components are separated based on their physiochemical properties.
- The elution process is monitored by a detector, and the data are analyzed to produce a chromatogram.
Importance of Selectivity
- Selectivity is the ability of SFC to separate two or more components from a complex mixture or sample based on their unique physiochemical properties.
- The selectivity, or specificity, of an SFC method is highly important, as it determines how well the components of a mixture can be separated and identified.
- By selecting a suitable stationary phase and mobile phase, the SFC method can have high selectivity and be tailored to separate target compounds precisely.
- Selectivity is essential, as it helps to improve detection limits, reduce the time to analyze complex mixtures, and provide better resolution between the various components in a sample.
- Selectivity is also utilized in preparative SFC, where the separated components of a comprehensive mixture can be isolated and recovered through various collection techniques such as trapping, fractionation, or recycling, thereby enhancing product yield.
Supercritical fluid chromatography is a versatile and powerful separation technique that can be used for a wide range of applications. The process involves the use of a stationary phase and a supercritical fluid mobile phase, which enables highly selective separation to occur. By selecting a suitable stationary phase and mobile phase and optimizing operating parameters, such as temperature and pressure, SFC can produce high-quality separations and be applied in the analysis and recovery of complex mixtures.
Markets Served
.png?width=218&name=phasex-logo-home%20(1).png)

 Process
Process