
Phasex works with established medical and biopharma companies that require the most stringent purity levels in their devices for exacting processes. Supercritical fluids extract toxic residues and residual monomers for the purification of medical products.

Feasibility Studies —These are short, fast-response feasibility studies to demonstrate that processing with supercritical fluids can achieve your desired goals, both technically and economically.
Contract R&D—R&D projects are designed to achieve your goals—from creating a new product, improving an existing one, recovering a by-product, or other purposes.
Toll Processing—Our ability to process 1,000 to 100,000 kg quantities enables you to evaluate supercritical fluids for their specific needs without investing early stage and high-risk capital
Technology Licensing—Phasex can develop technology for supercritical separations to be carried out as a part of a company’s own manufacturing train.
Increasingly stringent regulations have created the need for purer products in the medical industry. There has been increased concern about potentially migratable species that are present in many polymeric medical devices, such as brain shunts and aorta grafts that are surgically implanted in the body. Polymeric devices, especially those produced from silicone and polyester, typically contain several percent by-products and residual raw materials, which can be detrimental to the performance of the device and pose a serious health risk.
Devices manufactured from silicones can contain reaction byproducts in the end product because even highly purified silicone raw materials are known to react further during the device manufacture. This reaction produces additional byproducts directly in the finished part. Thus, a means of removing these byproducts while maintaining the integrity of the finished component is crucial. This is where Phasex excels.
Phasex has extracted medical polymers and final medical devices of their toxic residues without compromising overall product integrity. Supercritical carbon dioxide is extremely attractive for medical component extraction because of its technical attributes. CO2 swells polymers sufficiently to allow impurities to be extracted from the internal structure. The absorbed CO2 diffuses away when the pressure is reduced, and the component retains its original shapeand integrity
Organic solvents, such as hexane and methylene chloride, could be used to extract objectionable materials from polymeric-based medical components, but vestiges of these solvents are themselves difficult to remove without degrading or altering the characteristics of the device.
In some cases, the polymer can be purified before it is manufactured into a device. Raw polymer substrate used in the manufacturing of medical devices may contain a low MW constituent that can be deleterious to the body. Supercritical CO2 can purify the raw polymer prior to fabrication of the final medical device. In addition to the ability of supercritical CO2 to dissolve and extract impurities, the properties of supercritical CO2 are uniquely applicable for cleaning microporous surfaces of biomedical components because there are no surface tension limitations as do exist with liquid solvents
Some examples of case studies of work carried out by Phasex for the purification of medical and biopharma application are described below.
The National Heart, Lung, and Blood Institute (NHLBI) sponsored work with Phasex to apply supercritical fluid extraction to the purification of Poloxamer 188 (Pluronic® F68, the trade name NHLBI called it). In brief, the EO-PO-EO block copolymer produced by BASF was found by NHLBI researchers and other companies they had funded to be an excellent surfactant in the formulation of substitute blood. Unfortunately, some of the special batches of Pluronic® F68 produced for NHLBI were found to be contaminated by allyl end-group impurities, and NHLBI sought solutions to the problem. Work carried out with Phasex supercritical CO2 extraction over a wide range of conditions was shown to be an effective process to purify the surfactant.
Following the NHLBI study, one pharma company sought a source of purified, allyl-free Pluronic® F68 product that was to be purified from commercial Poloxamer 188. Phasex was able to develop, at lab-scale, a scalable process to extract commercial Poloxamer 188 into a purified allyl-free product. After only about four months of development, the optimized process was verified over a range of several parameters and scaled up in the Phasex plant in continuous countercurrent column three-shift operation, producing 78 pounds a day of purified polymer.
Alternative technologies and processes were available for consideration, yet one variation used a high temperature and another process used copious amounts of organic solvent. Operation of the process at high temperature led to concerns of polymer degradation. Also, operation with the solvent extraction process had questionable economics due to the cost of the solvent and subsequent waste disposal of the hazardous stream.
For their direct relevance to this discussion, Phasex presents GPC data for one of the EO-PO-glycerol feed materials that we purified. (We also demonstrated the versatility of the SC CO2 fractionation process, showing it was applicable to other medical/pharma polymeric feedstocks.)
Figure 1 is a GPC of the raw material; the allyls are present at ~ 6% concentration. Figure 2 shows the allyls that have been extracted, and Figure 3, the purified product. With this particular feed and ~ 6% of the incoming material comprising allyls, the yield of purified product was ~ 92%. (94% is theoretical.)
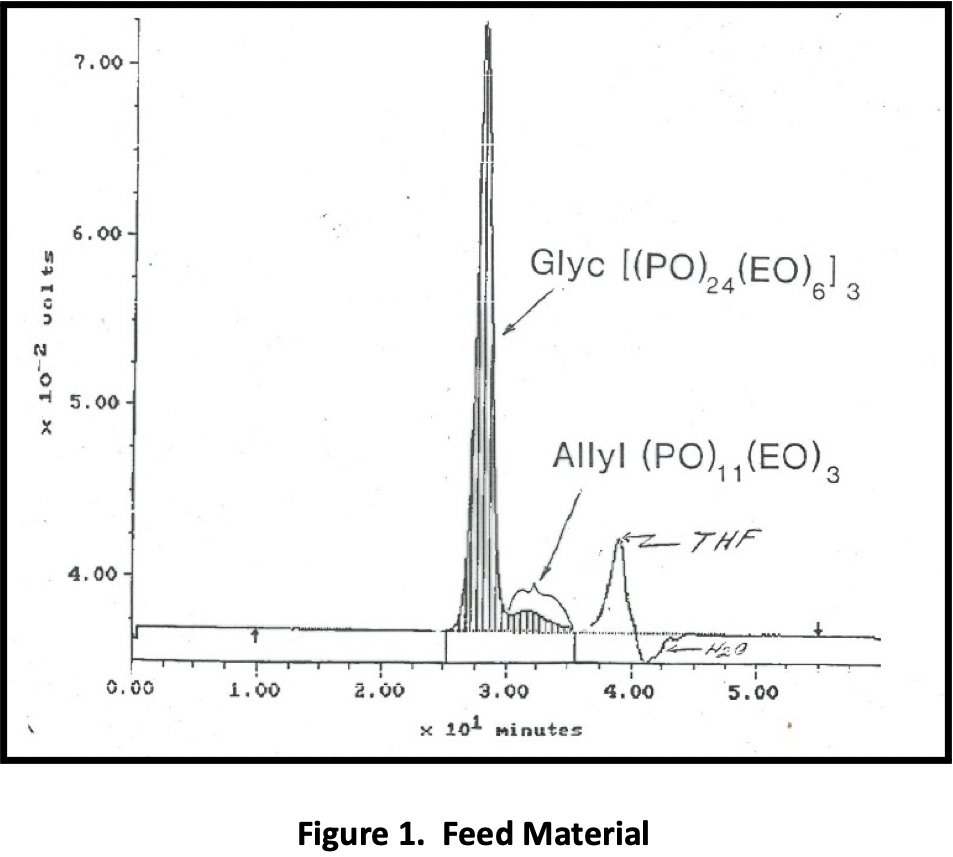
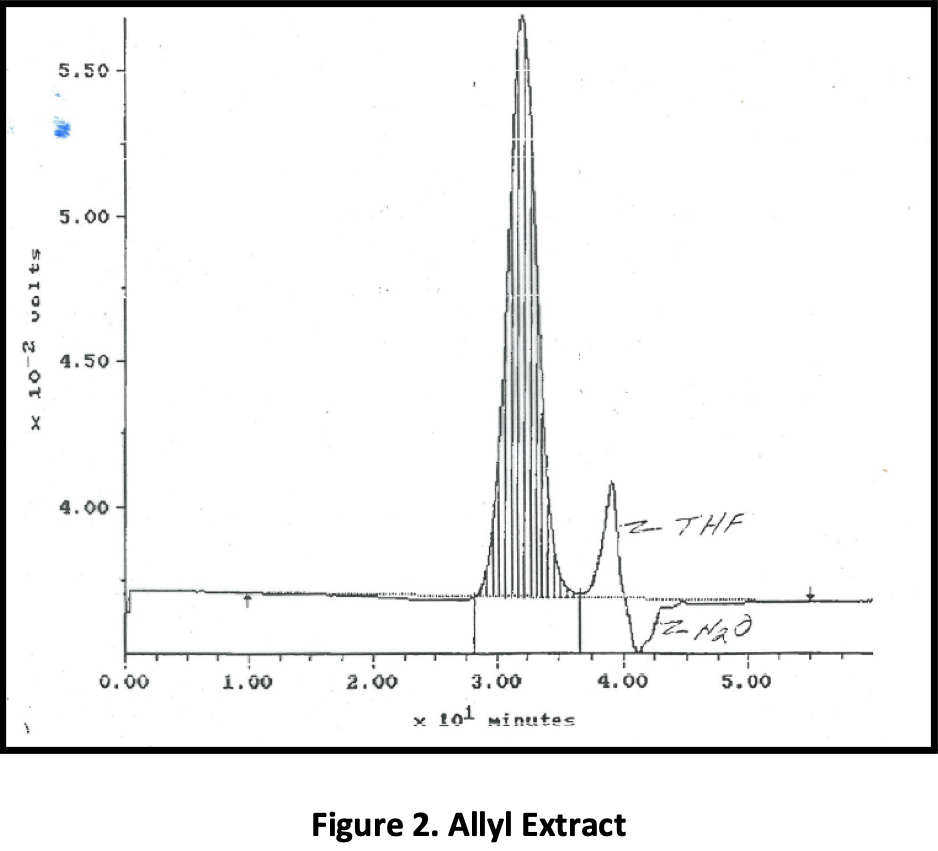
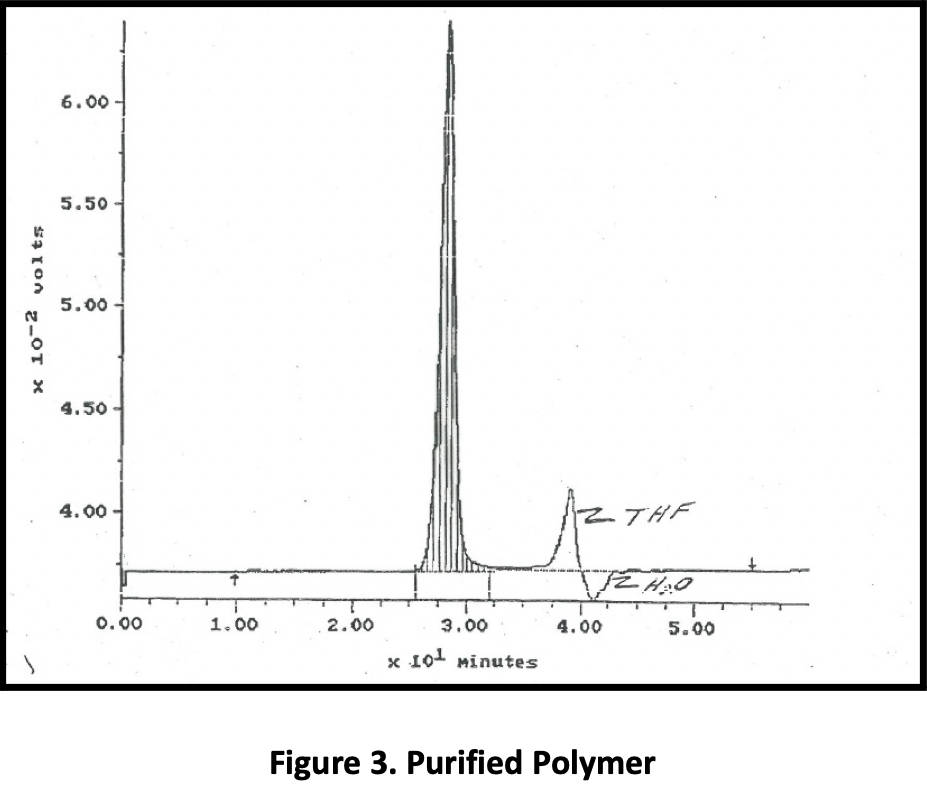
 One company approached Phasex to investigate the ability of SC CO2 to purify protein-based tissue scaffolds to be surgically implanted into the body. Surgical meshes and scaffolds are commonly used for a range of abdominal wall defect repair procedures, including reconstruction surgeries, bladder slings, and hernia repair. Although major complications with permanent, synthetic meshes are rare, there is growing concern about infection, difficulties associated with revision surgery utilizing current mesh products, and scar-plate formation. The company that engaged Phasex was using a silk-based surgical scaffold as a robust, long-term, bioresorbable biomaterial to address the limitations of today’s synthetic mesh products.
One company approached Phasex to investigate the ability of SC CO2 to purify protein-based tissue scaffolds to be surgically implanted into the body. Surgical meshes and scaffolds are commonly used for a range of abdominal wall defect repair procedures, including reconstruction surgeries, bladder slings, and hernia repair. Although major complications with permanent, synthetic meshes are rare, there is growing concern about infection, difficulties associated with revision surgery utilizing current mesh products, and scar-plate formation. The company that engaged Phasex was using a silk-based surgical scaffold as a robust, long-term, bioresorbable biomaterial to address the limitations of today’s synthetic mesh products.
The scaffold was required to be purified of contaminants and leachable oils that may have originated from its manufacturing process prior to end use. The concern was that solvent-based extraction (to purity the scaffold of residual oils and leachables) would, in turn, lead to residual levels of solvent with the implanted end product, and also, possibly denature the protein-based structure of the scaffold.
Phasex developed a CO2 purification process within two months’ time and was able to move to piloting and manufacturing with that same year. Through working with Phasex, the customer was able to generate purified tissue scaffolds for clinical trials without extensive outlay of funds to build a dedicated purification facility.
It should be noted that Phasex worked with the customer and an engineering firm to help the customer bring the purification process in-house at some later time, once the product gained foothold and wider use in the medical industry.
One medical tubing company approached Phasex for the purification of implantable siloxane-based hydrocephalus shunts. Hydrocephalus is a condition in which an accumulation of cerebrospinal fluid (CSF) occurs within the brain. This typically causes increased pressure inside the skull. Hydrocephalus can occur due to birth defects or be acquired later in life. Shunting is the most common treatment for anyone with hydrocephalus. Hydrocephalus shunting involves the implantation of two catheters and flow-control valve system to drain the excess accumulation of CSF from the brain’s ventricles (or the lumbar subarachnoid space) to another part of the body where it can be absorbed.
Phasex developed a CO2 purification process to remove residual low MW impurities created during the manufacturing process of the siloxane-based shunts. Similar to the scaffold project, Phasex was able to develop and validate a process within four months and move to cGMP production of quantities used for clinical studies. Phasex supported the customer’s production requirements for several years and then aided to bring the purification process in-house to the customer.
michele.mersicano@robertet.com
125 Flagship Drive
North Andover, MA 01845