This article appeared in the May 1998 issue of European Pharmaceutical Contractor (EPC) and is reproduced here with their permission. We hope it will serve as an easy-to-read introduction for those who are unfamiliar with the solvent properties of supercritical fluids. Although the examples given in the article are directed primarily to the pharmaceuticals and medical products industries, we think all readers will find the applications described of interest. If you desire more technical information, please contact us.
Supercritical Fluids: Their Proliferation in the Pharma Industry
by Val Krukonis
Supercritical fluids are finding increasing application in the pharma industry for the solution of difficult processing problems. Supercritical fluids exhibit a pressure-tunable dissolving power, they possess a liquid like density (and thus a high solvent strength), and their gas like transport properties allow facile extraction from dense botanical materials to be achieved. This unique combination of properties is ideally suited for developing processes for extracting, purifying, and recrystallizing fine chemicals and pharmaceuticals and producing new product forms that cannot be obtained by industry's more traditional processing technologies.
Background and Historical Perspective
The term 'supercritical fluid' describes a gas or liquid at conditions above its critical temperature and pressure - i.e., above the critical point. Drawing from physical chemistry texts, the critical point is located at the 'end' of the vapor pressure curve, and Figure 1 shows a generalized vapor pressure curve and its 'end'. The accented region in the figure denotes supercritical fluid space where many gases exhibit the propensity to dissolve materials.
At a meeting of the Royal Society (London) in 1879, two researchers, Hannay and Hogarth, reported that supercritical fluids have a pressure-dependent dissolving power-the higher the pressure, the higher their dissolving power (1). They described their work and summarized their findings as follows: We have the phenomenon of a solid dissolving in a gas, and when the solid is precipitated by reducing the pressure, it is brought down as a 'snow' in the gas. The researchers referred to supercritical fluids as gases, which, in fact, they are. In the interest of brevity, the term 'gas', or the abbreviation 'SCF' for supercritical fluids, will be used liberally throughout this paper.
The solubility behavior found by Hannay and Hogarth was not exploited until many, many years later, but it is of historical interest to relate some of the events surrounding their findings. There arose serious (but, as were the times, polite) controversy at the October 1879 society meeting: Some of the members who were present said, "Gases cannot dissolve solid compounds. The researchers must have erred and instead found solubility in superheated liquids." In other carefully planned and executed experiments the researchers did, however, substantiate their previous findings. Gases, in other words, supercritical fluids, could indeed dissolve many compounds.
A few years later, E. G. Buchner reported on his research in Zeitschrift fur Physicalische Chemie (1906). He became the first in a long line of researchers to measure the solubility of a model compound, naphthalene, in supercritical carbon dioxide (2). The Proceedings of the Royal Society (and other journals) describe much of the work during the early years of supercritical fluids activity, and naphthalene is still studied today for the information its solubility behavior presents to new researchers in the SCF field.
Solubility in Supercritical Fluids
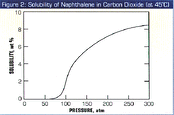 The solubility of naphthalene in supercritical carbon dioxide is shown in Figure 2. As one would expect, at low pressure its solubility is essentially nil. As the pressure of the gas is increased to above the critical pressure of carbon dioxide (which is 73 atm), the solubility rises, and for many compounds including naphthalene, the rise is often quite dramatic, as is seen in the Figure. For example, at 200 atm and 45°C, the solubility is 7%. The solubility behavior shown in figure 2 is the basis of almost all the supercritical fluid extraction/separation processes in operation throughout the world: Soluble components are extracted from a substrate by a high pressure gas, and the extracted components that have been dissolved in the gas are precipitated from the gas when the pressure is reduced, for example, across a pressure reduction valve.
The solubility of naphthalene in supercritical carbon dioxide is shown in Figure 2. As one would expect, at low pressure its solubility is essentially nil. As the pressure of the gas is increased to above the critical pressure of carbon dioxide (which is 73 atm), the solubility rises, and for many compounds including naphthalene, the rise is often quite dramatic, as is seen in the Figure. For example, at 200 atm and 45°C, the solubility is 7%. The solubility behavior shown in figure 2 is the basis of almost all the supercritical fluid extraction/separation processes in operation throughout the world: Soluble components are extracted from a substrate by a high pressure gas, and the extracted components that have been dissolved in the gas are precipitated from the gas when the pressure is reduced, for example, across a pressure reduction valve.
Starting in the 1960s, many research groups, primarily in Europe, and then later in the U.S., examined SCFs for developing 'advanced' extraction processes. European researchers emphasized extraction from botanical substrates, for example, spices, herbs, coffee, tea, and so on, using predominantly supercritical carbon dioxide, and by the 1980s there were several large SCF extraction processes in operation in Germany, the UK and the US, for decaffeinating coffee and tea and extracting flavors and essential oils from hops, spices, and herbs. As an example of size, a coffee decaffeination plant in Bremen processes more than 60,000,000 kg/year.
The major motivation for developing these SCF processes was the elimination of residual solvents in the products, especially methylene chloride, which had previously been used to decaffeinate coffee. Solvent residues in pharma and food products were becoming the focus of regulatory attention in the 1970s, and increasing regulatory attention is today being directed to solvent residues. Besides the elimination of solvent residues, there are also other advantages that accrue from employing supercritical fluids in coffee, spices, and herbs, i.e.: enhanced flavor and aroma characteristics that cannot be obtained by the traditional organic solvent extraction processes.
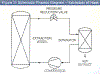 A schematic diagram of a generic supercritical fluid extraction process is shown in Figure 3 and, for concreteness, operation is described for the case of extracting hops, the extracted flavors being used in the brewing of beer. Compressed and pelleted hop flowers (called lupelones) are charged to the extraction vessel, which is subsequently secured for pressure operation. Carbon dioxide (from a reservoir not shown in the diagram) is pumped into the vessel, and after the temperature and pressure are adjusted to supercritical conditions of about 60°C and 300 atm, respectively, continuous flow of carbon dioxide is initiated. Hop flavors and lipids are extracted as the carbon dioxide flows through the charge of hops, and the solution (of carbon dioxide and flavors) is decreased in pressure to about 60 atm across the pressure reduction valve shown in Figure 3. Because the dissolving power of the carbon dioxide has been dramatically lowered, the flavors and lipids precipitate from the gas phase and collect in the separator; the carbon dioxide is recompressed and recycled to the extractor, and the process CO2 flow continues until all the flavors are extracted. The extraction vessel is then vented, the spent hops are removed, fresh pellets are charged to the vessel, and the process started again.
A schematic diagram of a generic supercritical fluid extraction process is shown in Figure 3 and, for concreteness, operation is described for the case of extracting hops, the extracted flavors being used in the brewing of beer. Compressed and pelleted hop flowers (called lupelones) are charged to the extraction vessel, which is subsequently secured for pressure operation. Carbon dioxide (from a reservoir not shown in the diagram) is pumped into the vessel, and after the temperature and pressure are adjusted to supercritical conditions of about 60°C and 300 atm, respectively, continuous flow of carbon dioxide is initiated. Hop flavors and lipids are extracted as the carbon dioxide flows through the charge of hops, and the solution (of carbon dioxide and flavors) is decreased in pressure to about 60 atm across the pressure reduction valve shown in Figure 3. Because the dissolving power of the carbon dioxide has been dramatically lowered, the flavors and lipids precipitate from the gas phase and collect in the separator; the carbon dioxide is recompressed and recycled to the extractor, and the process CO2 flow continues until all the flavors are extracted. The extraction vessel is then vented, the spent hops are removed, fresh pellets are charged to the vessel, and the process started again.
Besides the enhanced flavor characteristics and frequently higher yields associated with supercritical fluid extraction, some other technical and economic advantages reside in the use of carbon dioxide for the extraction of hop flavors. Organic solvents such as methylene chloride or hexane had previously been the solvents used for the extraction of hops. To obtain the concentrated flavors, it was necessary to distill off the organic solvents, and some of the top note aromas are lost during this step. Carbon dioxide produces a superior product because the top notes are not distilled off, and, as mentioned above, the issue of solvent residues, which is a constant spectre, is eliminated by the use of carbon dioxide.
In the 1980s other industrial applications of SCFs were being explored, for example, extraction of undesired byproducts from pharmaceuticals, purification of medical polymers, and separation of complex synthesis mixtures. 'Non-extractive' processes, were also being developed, the formation of ultra fine particles foremost among them. Currently the recrystallization of materials via SCF processing is under intense study at many pharma companies in Europe and the US. To be sure, there is continuing development on 'simple' extraction processes of residuals from medical polymers, of impurities from surfactants, and of active components or nutraceutical mixtures from botanical and biological substrates, and some of these supercritical CO2 processes are now in production, not at the huge production volume of coffee, tea, and hops, but at levels of 1,000 to 100,000 kgs per year or so, as is more characteristic of the medical products, fine chemicals, and pharmaceuticals industries.
Where are Supercritical Fluids Best Applied?
The author has widely communicated his view on using supercritical fluids for developing new products or processes - 'if it ain't broke, don't fix it' (3), in other words, don't force fit supercritical fluids into an application that is adequately handled by traditional industrial operations, such as solvent extraction, distillation, wiped film evaporation and the like. On the other hand, if research is fundamental in nature, for example, dealing with physico-chemical phenomena, thermodynamics, kinetic principles, and so on, it need not have industrial application, especially in the university system with its goals to teach, to instill curiosity, and to create knowledge. Applied industrial research should be sufficiently and justifiably motivated, especially in today's economic climate.
Where then, on both technical and economic grounds is the unique combination of properties and attributes of supercritical fluids most advantageously applied in developing improved processes and products?
-
Where environmental compliance pressures will soon require a change in the process
-
Where regulatory pressures will soon require a change in product purity
-
Where increased product performance will soon be required
-
Where an improved product can create a new market position
and where none of these can be achieved by industry's more traditional industrial processes.
It has been lamented by some that processing with supercritical fluids is not economical, and, unfortunately, the general impression does exist that supercritical fluids are associated with high processing costs; however, supercritical fluid decaffeinated coffee available at competitive market prices (with organic solvent decaffeinated coffee) certainly would contradict this impression. The misassociation of high cost with supercritical fluid processes has undoubtedly derived from the fate of several widely publicized (but ill-advised) studies in the late 1970s, whose lack of industrial viability was attributed solely to a high processing cost when, in actuality, there were technical limitations (that were not described). As with the 'if it ain't broke' discussion above, the author suggests that a case-by-case evaluation of economic viability be made early in any new application development. Supercritical fluids are frequently excellent solvents technically, with far ranging applications to many purification problems, but they may not be economically viable in each case.
Three examples of successful applications of supercritical fluids in the pharma, fine chemicals, and medical products industries are described below.
Recrystallization of Pharmaceuticals
Some pharma compounds are difficult to micronize by conventional grinding or jet milling. For example, materials that have a low (less that 60°C) melting point, or that are waxy, cannot be ground or milled to fine size (less than 1 or 2 microns), because they will smear or form amorphous and size unstable particles. Two supercritical fluid processes have been developed in response to these difficulties. In one, a supercritical fluid is employed as a solvent to dissolve a pharma compound, then, by pressure decrease, cause precipitation; in the other, the gas acts as an anti solvent, causing recrystallization from a liquid solution because of a solubility decrease when the gas and liquid solvent contract.
Recall Hannay and Hogarth's statement on the formation of 'snow'. Assuredly, the snow was of a size different from that of the parent material, and their observations of 100 years ago are the basis of the particle formation process termed RESS (rapid expansion of supercritical solutions). RESS is employed with SCF soluble compounds. If a pharma compound is not soluble in a gas (and many very polar compounds are not), the gas can be used an anti solvent, causing recrystallization when the gas and an organic solvent solution are admixed. The process, termed GAS (gas anti solvent) recrystallization, exploits the miscibility of carbon dioxide with virtually all laboratory and industrial solvents. With a gas anti solvent, the rapidity and intimacy of mixing far exceeds what liquid anti solvents can achieve, and thus GAS recrystallization can achieve narrow particle size distribution in the 100s of nanometer range.
 The advantages of both processes reside in the literally millisecond timescale for pressure reduction or admixing of solution and gas, which creates a high supersaturation ratio, thus resulting in the formation of ultrafine particles of narrow size range. As related earlier, market driven motivations for applying supercritical fluids to recrystallization opportunities will enhance the probability of an economic success; for example, creation of a new pharma product that be delivered via inhalation, instead of parenterally, would certainly justify the application of supercritical fluids, especially if traditional jet milling can not achieve the desired product characteristics.
The advantages of both processes reside in the literally millisecond timescale for pressure reduction or admixing of solution and gas, which creates a high supersaturation ratio, thus resulting in the formation of ultrafine particles of narrow size range. As related earlier, market driven motivations for applying supercritical fluids to recrystallization opportunities will enhance the probability of an economic success; for example, creation of a new pharma product that be delivered via inhalation, instead of parenterally, would certainly justify the application of supercritical fluids, especially if traditional jet milling can not achieve the desired product characteristics.
Figure 4 shows comparative photomicrographs of one compound, ßzlig-pregnanolone, recrystallized from supercritical carbon dioxide. The parent material ranges in size from about 2 to 15 microns, and the supercritical fluid processed (by RESS) material ranges from 1 to 2 microns. The photomicrographs exemplify the capabilities of supercritical recrystallization, and as related earlier, many pharma companies in the US and Europe are evaluating supercritical fluids for forming nano-sized pharma compounds in the development of advanced delivery formulations.
Purifications of Surfacants
Liquid and solid surfactants are used in the formulation of many pharma products. These surfactants are usually the same ones that have been produced for less demanding, but very high volume applications, ranging from food products to cleaning solutions and floor wax. Many surfactants suffer from color, odor, byproducts or other limitations and, usually, these surfactants have, for pharma applications, been processed to the best purity levels achievable with industry's traditional technologies. Increasingly, supercritical fluid extraction is demonstrating the ability to purify several types of surfactants of importance for pharma formulations.
 As an example of the separation of color species, Figure 5 shows samples of two surfactants and the corresponding SCF purified materials. The color of the parent materials is a result of polymerizing reactions that occur during synthesis. Because the vapor pressure of the materials is very low, they cannot be distilled to improve their color. Supercritical carbon dioxide separates materials on the basis of solubility, not vapor pressure, and thus it can purify heat sensitive materials that cannot be processed by distillation or very low vapor pressure.
As an example of the separation of color species, Figure 5 shows samples of two surfactants and the corresponding SCF purified materials. The color of the parent materials is a result of polymerizing reactions that occur during synthesis. Because the vapor pressure of the materials is very low, they cannot be distilled to improve their color. Supercritical carbon dioxide separates materials on the basis of solubility, not vapor pressure, and thus it can purify heat sensitive materials that cannot be processed by distillation or very low vapor pressure.
Extraction of Byproducts from Polymer Components
Over the past five years, there has been increased scrutiny of potentially migratable species that are present in polymeric devices that are inserted or surgically implanted in the body, for example, urinary catheters, brain shunts, aorta grafts and the like. The polymers, especially if they are silicone or polyester based, normally contain several percent residual byproducts and raw materials. Sometimes the objectionable components can be removed from the polymer before it is manufactured into the device, and sometimes they cannot, especially if the polymer is silicone based. Silicone raw materials, for example, normally react further during the manufacture of the device, the reaction producing the byproducts directly in the finished part.
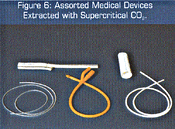 Organic solvents, such as hexane and methylene chloride, can usually dissolve and extract objectionable components, but vestiges of these solvents are themselves often difficult to remove without degrading or altering the characteristics of the polymer or device. The attributes of supercritical fluids, especially CO2, are again extremely attractive in the polymer extraction application. Carbon dioxide swells polymers sufficiently to allow the interior volumes to be reached in order to dissolve and carry away the undesired materials and, when the pressure on the part is reduced, all the carbon dioxide is removed. Figure 6 shows selected medical components that are currently being processed with supercritical fluids to remove undesired species.
Organic solvents, such as hexane and methylene chloride, can usually dissolve and extract objectionable components, but vestiges of these solvents are themselves often difficult to remove without degrading or altering the characteristics of the polymer or device. The attributes of supercritical fluids, especially CO2, are again extremely attractive in the polymer extraction application. Carbon dioxide swells polymers sufficiently to allow the interior volumes to be reached in order to dissolve and carry away the undesired materials and, when the pressure on the part is reduced, all the carbon dioxide is removed. Figure 6 shows selected medical components that are currently being processed with supercritical fluids to remove undesired species.
Concluding Remarks
When Professor Andrews of Queens College (4) determined the critical point of carbon dioxide, he probably did not know of its solvent properties, and Hannay and Hogarth (and Buchner, and scores of others in the early 1900s) could not have envisaged that a huge plant would be built in 1978 (in Bremen), decaffeinating coffee with supercritical carbon dioxide in a process that eliminates regulatory concerns about solvent residues, environmental concerns about ozone depletion and hydrocarbon emissions, and worker safety concerns (because carbon dioxide in non-toxic and non-flammable), while simultaneously producing a product with superior taste and at a cost competitive with the standard organic solvent-based processes.
The pharma industry (as well as others) is starting to recognize the technical, regulatory, and market attributes of supercritical fluids, and is increasingly applying them to the solution of difficult problems. It is opined that the 21st Century will see many processes that have been developed to exploit the properties of supercritical fluids. The caution, 'if it ain't broke, don't fix it', should guide each evaluation. But if it is 'broke', maybe supercritical fluids can fix it.
References
(1) Hannay, J.B. and Hogarth, J., On the Solubility of Solids in Gases, Proc. Roy. Soc., (London). 29:324. 1879
(2) Buchner, E.G., Die beschrankte Mischbarkeit von Flussigkeiten das System Diphenyamin und Kohlensaure, Z. Phys. Chem., 56:257. 1906
(3) Krukonis, V., Brunner, G., and Perrut, M., Industrial Operations with Supercritical Fluids: Current Processes and Perspectives on the Future, Proceedings 3rd International Symposium on Supercritical Fluids, Tome 1. p1. Strasbourg, 17th October 1994
(4) Andrews, T., The Bakerian Lecture - On the Gaseous State of Matter., Proc. Roy. Soc., (London). 24:455. 1875
.png?width=218&name=phasex-logo-home%20(1).png)


