Understanding the SCF Process and What that Means for Your Ingredients
While the supercritical fluid process relies on an intimate understanding of the science behind it, grasping the concept is far easier. A supercritical fluid can be defined best with as follows: every compound has a critical point—the pressure and temperature at which its liquid and vapor phases become identical, and thus, exhibits zero surface tension.
This gas or liquid at pressure and temperature conditions that are above its critical point is termed a supercritical fluid (SCF). Every substance on earth exhibits a similar behavior as shown on P-T diagram below.
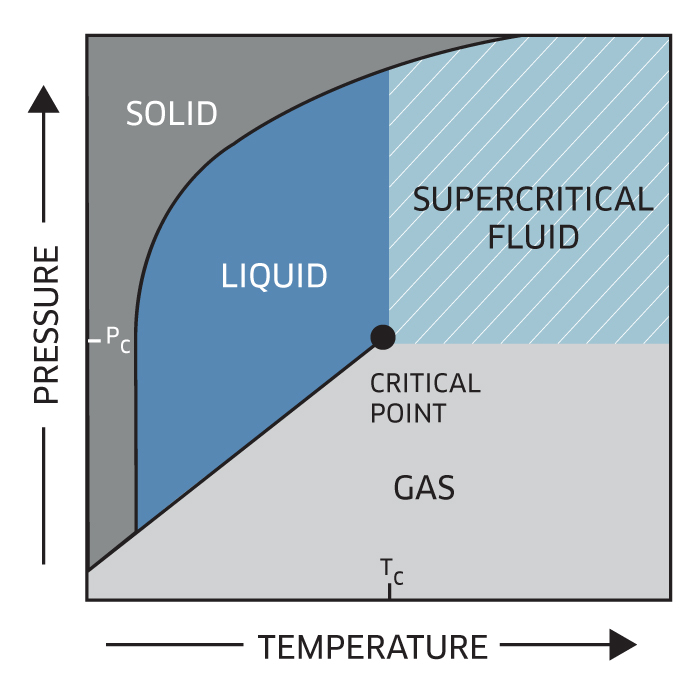
More than 100 years ago, it was found that supercritical fluids can dissolve a wide range of organic compounds, such as pharmaceuticals, essential oils, polymers, lubricants. The most widely known uses of supercritical fluids is in the application of hops extraction or the decaffeination of coffee and tea. Supercritical fluids exhibit a pressure-dependent dissolving power, and these solubility properties have been applied to the development of processes to extract, purify, and fractionate a wide variety of materials.
The most prevalent gas used in supercritical processing is CO2. Supercritical CO2 offers the ability to extract and purify various substrates across a wide range of industries without the deleterious effects of solvent processing. What this means is there is no solvent residue, no volatile organic compounds (VOC) concerns, no flammability concerns, and all achieved with clean and environmentally conscious processing.
Why Are Supercritical Fluids Important to Me?
Industry has been faced with increasing demands on products and processes: increasingly stringent regulations on liquid solvent use in the workplace, on residual solvents in products, and on solvent emissions. These factors have motivated the application of supercritical fluids, especially supercritical CO2, to the development of processes to satisfy these requirements, especially where traditional solvents and processing methods fall short.
The pressure-dependent dissolving power of supercritical fluids can allow the user to selectively remove impurities, purify and concentrate actives, and clean substrates to levels not seen with traditional solvent extraction. The high-diffusivity and non-existent surface tension of supercritical fluids can result in an extraction process that can penetrate and find accessibility into micropores structures to a level that is not attainable with industrial solvents.
The zero surface tension phenomena of supercritical CO2 have been exploited commercially to:
- Infused anti-microbial, anti-bacterial, and preservative agents into lumber (Supertrae or “Superwood”) to make environmentally friendly pressure-treated wood
- Extraction of porogens from polymeric matrices for lithium battery manufacturing processes
- Critical point drying of aerogels to be used as superior insulation devices
- Infusion of organo-metallic compounds into nano-structure composites
The particular attributes of supercritical CO2 to be exploited by industry:
- USDA organic extraction process
- Mild operating temperature for a “gentler” extraction process compared to distillation
- GRAS (generally recognized as safe) processing method
- No solvent residue, no flammability concern, no VOC concerns
- Environmentally benign operation
Where Can Supercritical Fluids Be Applied in Industry?
Supercritical fluid processing has been applied in industry for decades at commercial scale in the decaffeination of tea/coffee and the extraction of hops flavoring. SCF toll processing and extraction serves the natural products extracts markets, medical/biopharma, and industrial/polymers markets for their respective needs.
In recent years, there has been an explosion of numerous supercritical CO2 applications due to their unique ability to solve difficult separation problems and their benign nature towards the environment. Applications to be considered for use with supercritical fluids are:
- Extraction and concentration of actives from botanical and natural substrates
- Purification of heat-labile compounds and reactive monomers
- Fractionation of polymers by molecular weight (MW) and molecular structure
- Cleaning of medical devices and polymeric resins
- Infusion of micropores membranes and nanostructures with actives
Exploring SCF for Your Business
As pioneers in the industry with 40 years of expertise, Phasex is the pre-eminent leader in supercritical fluid CO2 extraction. We are a full-service company providing feasibility studies, R&D, toll processing, and technology licensing to industry and government. If you’d like to learn more about supercritical fluids, we’ve written a more in-depth discussion in our SCF primer, which you can find here.
Curious about how SCF CO2 processing can help you meet your goals? We’re happy to discuss a feasibility assessment with you about your supercritical fluid needs. Or contact us now if you have any questions we can answer.
.png?width=218&name=phasex-logo-home%20(1).png)


